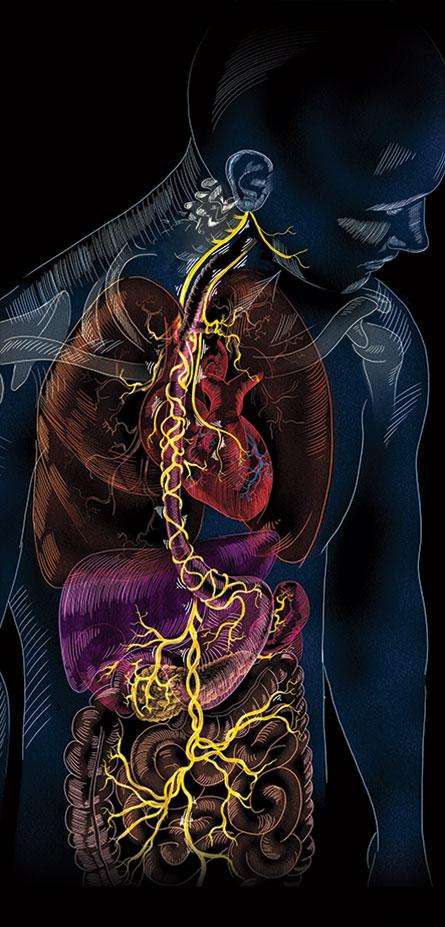
Vagus nerve, the most important &
longest nerve in your body.
Non-invasive vagus nerve stimulation (nVNS) is a treatment approach that involves stimulating the vagus nerve non-invasively, usually through the use of a device that delivers electrical impulses to the nerve. nVNS is an experimental treatment that is being studied for a variety of conditions, including depression, anxiety, and chronic pain.
Research on nVNS has been ongoing for several years (since 1979), and the results of studies have been mixed. Some studies have suggested that nVNS may be effective in reducing symptoms of depression and anxiety, while others have not found significant improvements.
One of the challenges of studying nVNS is that it is not clear how the treatment works. It is thought that nVNS may work by activating certain pathways in the brain that are involved in mood regulation, but the exact mechanisms are not well understood.
Overall, the state of research on nVNS is still in the early stages, and more research is needed to fully understand the effectiveness and mechanisms of the treatment. While some studies have suggested that nVNS may be helpful for certain conditions, more research is needed to confirm these findings and to determine the optimal use of nVNS in clinical practice.
As an emerging neuromodulation therapy, transcutaneous auricular vagus nerve stimulation (taVNS) has been proven to be safe and effective for epilepsy, major depressive disorders, insomnia, glucose metabolic disorders, pain, stroke, post stroke rehabilitation, anxiety, fear, cognitive impairment, cardiovascular disorders, tinnitus, Prader-Willi Syndrome, and COVID-19.
Areas covered: Although the history of taVNS is only two decades, the devices carrying taVNS technique have been constantly updated. Especially in recent years, the development of taVNS devices has presented a new trend. To conclude, the development of taVNS devices has entered a new era, thus the update speed and quality of taVNS devices will be considerably improved in the future.
The Polar Bear Foundation will make sure it happens.
Clinical Trials
Phase #1 : 2 hours per day
Phase #2 : 8 hours per day (Night & Day)
Prime Goal: Smart Device will be supplied to Health departments.
-----
Once, we're done, everyone will have access to a smart device, with a custom stimulation protocol that will be done from home.



